To best describe corrosion, let’s start with the most common type, rust. We all know rust, but to understand rust, we have to go back to the very beginning. Iron ore has a chemical composition of two iron atoms bonded with three oxygen atoms. As it is mined out of the ground, it’s a brownish-red powder useless to us. But by refining, purifying, and smelting, we create iron, which is useful. We can use it as plain iron, or we can process it further and combine it with other elements to get different types of steel.
Electrochemical Reactions
Iron left out in the rain results in a specific kind of corrosion. It’s called an electrochemical reaction, meaning there is an electrical change. Here’s how that works:
For two iron atoms to really interlock with three oxygen atoms and make iron, they have to share some electrons, which releases a few electrons. Since electricity is just a flow of electrons, those free electrons become a little bit of electricity when the chemical change takes place.
 Remember the iron wants to corrode into iron oxide because that is its natural, most stable state. And all it needs for this to take place is oxygen. Water is a supply of oxygen, so iron rusts fastest when it gets wet. You knew that already but now you know why. And that same scenario applies to aluminum and aluminum oxide. Those are the deep, dark secrets of corrosion as they apply to metals. Those are also the basics of an electrochemical reaction, which is known as galvanic corrosion. All galvanic corrosion is an electrical reaction. Not all electrochemical reactions, however, are galvanic corrosion.
Remember the iron wants to corrode into iron oxide because that is its natural, most stable state. And all it needs for this to take place is oxygen. Water is a supply of oxygen, so iron rusts fastest when it gets wet. You knew that already but now you know why. And that same scenario applies to aluminum and aluminum oxide. Those are the deep, dark secrets of corrosion as they apply to metals. Those are also the basics of an electrochemical reaction, which is known as galvanic corrosion. All galvanic corrosion is an electrical reaction. Not all electrochemical reactions, however, are galvanic corrosion.Galvanic Corrosion
Galvanic corrosion is an electrochemical reaction between two or more different metals. The metals must be different because one must be more chemically active (or less stable) than the others for a reaction to take place. When we talk about galvanic corrosion, we’re talking about electrical exchange. All metals have electrical potential because all atoms have electrons, which have an electrochemical charge.
Galvanic corrosion of the more chemically active metal can occur whenever two or more dissimilar metals that are "grounded" (connected by actually touching each other, or through a wire or metal part) are immersed in a conductive solution (any liquid that can transfer electricity). Anything but pure water is conductive. Saltwater, freshwater with high mineral content, and polluted freshwater are very conductive, and conductivity goes up with water temperature. That’s one reason why boats in Florida experience more corrosion than boats in Maine.
The simplest example of galvanic corrosion, and the most applicable, is an aluminum lower unit with a stainless steel propeller. The aluminum is the more chemically active metal (the anode), and the stainless steel is the less chemically active metal (the cathode). Several things happen at the same time:
At the Anode
1. Electrons flow from the anode, the metal that is more chemically active (the aluminum drive unit), via the external conducting path to the cathode, the metal that is less chemically active (the stainless steel prop).
2. When this happens, the more chemically active metal atoms become ions (an atom with one or more electrons either missing or added) and break away into the water, where they can bond to oxygen ions, with which they can share electrons and produce aluminum oxide. This is the same process iron ions go through when combining with oxygen ions in water to form iron oxide.
3. The newly formed aluminum oxide molecules either drift away in the water or settle on the surface of the aluminum. Your lower unit is literally dissolving through galvanic corrosion.
At the Cathode
1. Electrons are accepted from the anode; however, they cannot simply accumulate, they react with ions in the electrolyte.
2. The resulting hydroxide ion is alkaline, and makes the electrolyte alkaline in the area of the cathode. This detail is especially important for wooden boats, as an alkaline solution will attack cellulose (i.e. wood).
It's important to understand that for each positive metallic ion released at the anode, electrons in the cathode react to form a negative ion in the electrolyte. Electrically the anodic and cathodic reactions must be equivalent. Increases or decreases in the rate of the cathodic reaction will have a corresponding increase or decrease on the anodic reaction. This is a basic fact in understanding and controlling corrosion. This fact can also be demonstrated by the effect of size ratios between anodes and cathodes. If there is a very large anode connected to a small cathode, the anode will corrode very slowly. However, if a very large cathode is connected to a small anode, the anode will corrode very rapidly. Marine drive components have many aluminum parts. If you do not control galvanic corrosion, over time the aluminum will corrode away.
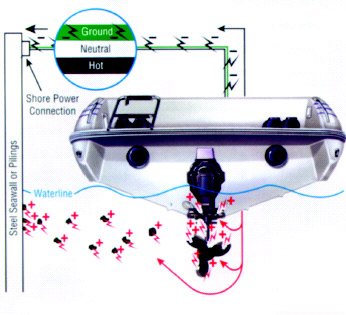
Galvanic corrosion can also occur without any stainless steel components on your boat. For example, you have an aluminum drive unit and an aluminum propeller, but you dock at a pier with steel pilings or a steel seawall, then plug into shorepower. The ground wire, which is grounded, connects your aluminum components with the submerged steel because the steel is also grounded. Considering the mass of a seawall or even a single piling, your drive and propeller can sustain serious damage. This damage could be prevented with a galvanic isolator.
What to Look For
The first sign of galvanic corrosion is paint blistering (starting on sharp edges) below the water line—a white powdery substance forms on the exposed metal areas. As the corrosion continues, the exposed metal areas will become deeply pitted, as the metal is actually eaten away.

Typical signs of corrosion on marine lower drive units and propellers include blistering paint and the formation of a white powdery substance on the exposed metal areas
Galvanic corrosion of aluminum drive units—or any underwater aluminum on your boat—is accelerated by attaching stainless steel components like propellers, trim planes (if connected to engine ground), and aftermarket steering aids. In doing this, you have introduced a dissimilar metal to which electrons from your drive unit will follow. Another condition that will increase the speed or intensity of galvanic corrosion is the removal or reduction in surface area of sacrificial anodes. But you don’t need stainless steel components for galvanic corrosion to take place. Galvanic corrosion continually affects all underwater aluminum, but at a reduced rate when no dissimilar metals are connected to your aluminum parts. When in contact with an electrolyte, most metals form small anodes and cathodes on their surfaces due to such things as alloy segregation, impurities, or cold working.
We have used stainless steel (cathode) and aluminum (anode) in this discussion as an example, however other metals coupled with aluminum also produce galvanic corrosion cells. For example, zinc connected to aluminum will form a corrosion cell, but in this case, the aluminum becomes the cathode and the zinc (anode) corrodes. One of the worst couples with an aluminum drive would be connecting it with copper or a copper alloy (bronze). Another cause of galvanic corrosion is the shorepower hookup. When you plug in, you tie your aluminum drive unit to other boats using shorepower through the green grounding lead. Your aluminum drive unit is now part of a large galvanic cell (a battery) interconnected with onshore metal that is in the water—as well as other boats—and corrosion may be greatly accelerated.
Article courtesy of Quicksilver Marine.
Leopad Group a leading provider of corrosion
protection services ranges from the scope of blasting and painting, insulation,
thermal spray application, passive fire protection, refractory and other
services such as scaffolding, cable tray systems and cathode protection.
We are a
Malaysian company with close to 3000 staff and over 10 offices and fabrication
yards throughout the country. Leopad Group is dedicated to being the market
leader for corrosion protection and provide the highest standards in the
industry with the convenience of providing multi-disciplinary services through
a single point of contact.
For further
enquiries on our services, please contact our Business Development Department
at +603-22600200 , website www.leopad.com or email at hq@leopad.com




