In North America, the only standard that adequately
addresses serious environmental exposures and the longevity of intumescents is
UL1709 (Standard for Safety for Rapid Rise Fire Tests of Protection Materials
for Structural Steel) (2). Unfortunately, this standard only covers fire
testing of structural steel columns against the hydrocarbon
time-versus-temperature curve, which essentially restricts its use to fire
protection of exterior steel structures in the oil and petrochemical industries
(a minority of applications for intumescent products). In fact, testing against
UL1709 can deter manufacturers of intumescents, and for good reasons, which are
described later.
UL1709 contains a very tough set of tests, as it
should, considering the rigorous applications for exterior hydrocarbon fire
protection and the enormous risk potential for refineries and chemical plants.
This test regime does nothing for interior products because no one has tested
common interior passive fire-protection products to UL1709. One reason is that
endothermic coatings such as intumescents generally contain large
concentrations of epoxy coatings. Consequently, an unacceptably high amount of
smoke is generated when the coating is first exposed to fire, and such a large
fuel contribution violates building codes across North America.
Although other methods for testing intumescent
materials are available, the best and most scientifically sound bench-scale
testing is from DIBt. Under the DIBt approval guidelines, building materials
are tested for interior or exterior applications. DIBt-approved firestopping,
fireproofing, and gasketing products are available in North America;
manufacturers include 3M (intumescent firestops) and Nullifire (a thin-film
intumescent spray fireproofing product).
Committees from UL and UL of Canada (Toronto) are
currently considering mandating the use of a modified version of the DIBt
method for bench-scale testing of intumescent products used in firestopping
applications. Neither U.S. nor Canadian manufacturers are enthusiastic about an
environmental exposure mandate. However, manufacturer due diligence and
providing useful and reliable data to the end user are the real issues because
it is the costly environmental exposure testing as well as practical
performance that causes some intumescents to fail.
Assessing
the intumescent market
The largest market for intumescents is the industrial
exterior spray fireproofing market. All manner of passive fire protection
products compete for this market, including intumescents, other endothermic
products, cementitious plasters, fibrous plasters, fibrous wraps, and cast
concrete, as well as active fire protection products, such as the type of
sprinkler systems used to protect liquefied petroleum gas (LPG) containers.
Let us look at a situation in which problems
associated with rival products can be solved by intumescents: petrochemical
plants, which contain process pipe bridges (structural steel racks for the
purpose of holding up process piping), vessel skirts (round steel sheet
structures, which support a vessel above), and spherical or cylindrical LPG
containers.
There is a definite demarcation line within
petrochemical facilities in terms of the importance given to (and willingness
to part with funds to safeguard) pipe bridges and vessel skirts compared with
LPG containers. Without vigilant enforcement measures, many above-ground LPG
containers remain unprotected and thus subject to fire exposure in case of a
flammable hydrocarbon spill. Facility owners who do pay to fireproof their LPG
vessels are more likely, if aware of all technical aspects and expenses, to
choose an intumescent or other endothermic product, rather than a cementitious
or fibrous plaster. There are several reasons for this; one is longevity.
There is considerable variation in reinforcement
factors and inherent flexibility between cementitious plaster products used for
spray fireproofing (7). Plaster delamination and the corrosion of the steel
mesh used to reinforce the plaster have in some cases caused the spray
fireproofing to become dislodged and fall off. Common factors known in
inorganic chemistry and in the concrete industry contribute to such events.
Omitting the exterior waterproofing membrane or the priming layer permits
weathering to occur, causing the cement-bound plaster to drop in pH value. This
reduces the corrosion protection of the reinforcing mesh, which starts to rust,
expand, and thus potentially damage the vulnerable plaster. This effect is
especially pronounced in installations near the ocean, where the salt spray
accelerates corrosion.
Experience has shown that because of faulty dew-point
calculations and insufficient investment in quality materials and installation,
fibrous plasters can become soaked with water and then freeze and delaminate.
The owner of a petrochemical facility may find that the absolute lowest price
is not the best tool for cost effectiveness in the long run.
Intumescent and endothermic products for this
application circumvent the problems associated with the cementitious and
fibrous plasters, for the most part. There have been cases of misapplication of
intumescents (e.g., mixing incorrect proportions of ingredients) leading to the
sliding off and total replacement of the product during the initial application.
Intumescent coatings have also been known to delaminate; however, these are
exceptional cases. One should use caution to choose a competent contractor.
Intumescent and endothermic products are supplied in an epoxy paint base, so
they are inherently corrosion-inhibiting. They are also much more likely to
stretch and move with a sphere as it is being emptied and filled and undergoing
weather changes. There can be no water or chloride penetration, and there is no
cement to suffer from corrosive effects. Intumescent and endothermic products
are significantly more expensive per square meter installed than fibrous and
cementitious plasters. In light of the technical aspects, intumescent and
endothermic products are worth the extra money, particularly when qualified to
the environmental criteria of UL1709 or DIBt.
At present, there is no shortage of vendors for
intumescent products, certified or not. If you are a manufacturer of passive
fire protection products, it is certainly wise to be up-to-date on DIBt standards.
I recommend qualifying your products with this method and obtaining a DIBt
approval regardless of where your product may be sold, simply to prove due
diligence as a manufacturer. If you are purchasing intumescents for your
facility or for resale or you are specifying them for use in someone else’s
facility, requesting a current DIBt approval and a system qualified to UL1709
is prudent. Beware that for each standard, not every exposure is mandatory.
Source: Achim Hering http://pubs.acs.org/subscribe/archive/ci/31/i05/html/05hering_new.html
Leopad Group a leading provider of corrosion
protection services ranges from the scope of blasting and painting, insulation,
thermal spray application, passive fire protection, refractory and other
services such as scaffolding, cable tray systems and cathode protection.
We are a Malaysian company with close to 3000 staff and over 10
offices and fabrication yards throughout the country. Leopad Group is dedicated
to being the market leader for corrosion protection and provide the highest
standards in the industry with the convenience of providing multi-disciplinary
services through a single point of contact.
For further enquiries on our services, please contact our Business
Development Department at +603-22600200 , website www.leopad.com

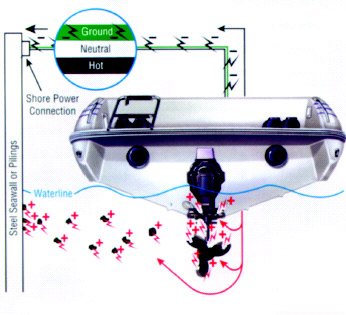
 stainless (nonrusting) is its formation of a thin, tightly adhering surface layer of chrome oxide. If this surface is deprived of oxygen, the oxide layer breaks down and the stainless steel will rust just like plain steel. In other words, stainless steel is only stainless when it has access to oxygen. In a crevice where there is moisture depleted of oxygen, stainless steel rusts. The simplest prevention for this condition is to seal out the moisture or clean off any deposits.
stainless (nonrusting) is its formation of a thin, tightly adhering surface layer of chrome oxide. If this surface is deprived of oxygen, the oxide layer breaks down and the stainless steel will rust just like plain steel. In other words, stainless steel is only stainless when it has access to oxygen. In a crevice where there is moisture depleted of oxygen, stainless steel rusts. The simplest prevention for this condition is to seal out the moisture or clean off any deposits.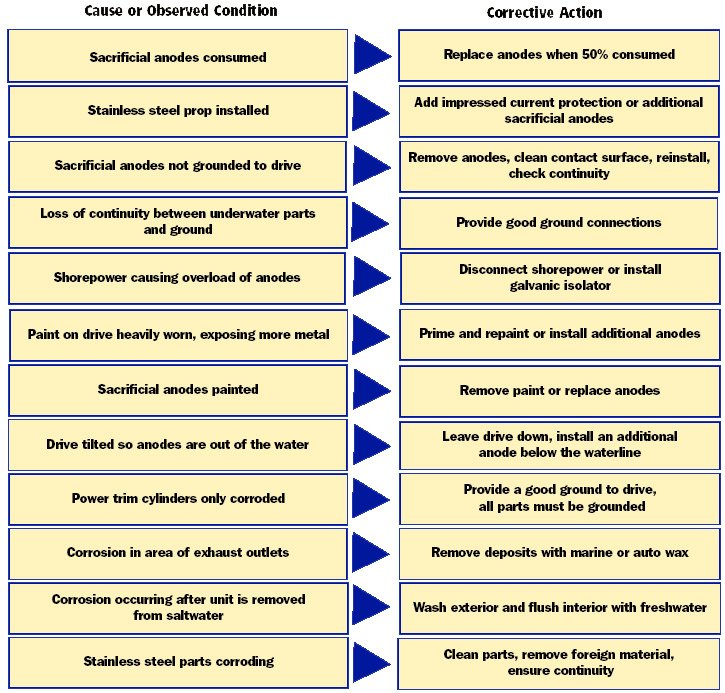
 Remember the iron wants to corrode into iron oxide because that is its natural, most stable state. And all it needs for this to take place is oxygen. Water is a supply of oxygen, so iron rusts fastest when it gets wet. You knew that already but now you know why. And that same scenario applies to aluminum and aluminum oxide. Those are the deep, dark secrets of corrosion as they apply to metals. Those are also the basics of an electrochemical reaction, which is known as galvanic corrosion. All galvanic corrosion is an electrical reaction. Not all electrochemical reactions, however, are galvanic corrosion.
Remember the iron wants to corrode into iron oxide because that is its natural, most stable state. And all it needs for this to take place is oxygen. Water is a supply of oxygen, so iron rusts fastest when it gets wet. You knew that already but now you know why. And that same scenario applies to aluminum and aluminum oxide. Those are the deep, dark secrets of corrosion as they apply to metals. Those are also the basics of an electrochemical reaction, which is known as galvanic corrosion. All galvanic corrosion is an electrical reaction. Not all electrochemical reactions, however, are galvanic corrosion.