The first practical use of cathodic protection is generally credited to Sir Humphrey Davy in the 1820s. Davy’s advice was sought by the Royal Navy in investigating the corrosion of copper sheeting used for cladding the hulls of naval vessels. Davy found that he could preserve copper in sea water by the attachment of small quantities of iron or zinc; the copper became, as Davy put it, “cathodically protected”.
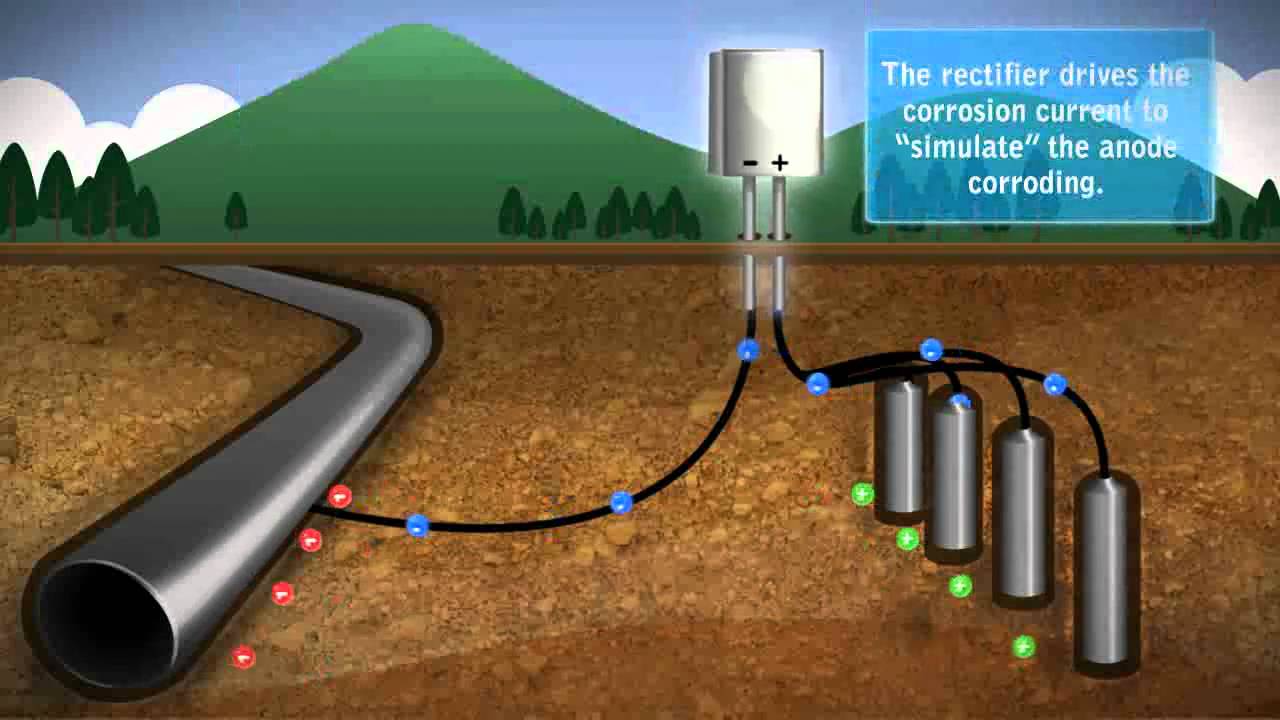 The most rapid development of cathodic-protection systems was made in the United States of America to meet the requirements of the rapidly expanding oil and natural gas industry which wanted to benefit from the advantages of using thin-walled steel pipes for underground transmission. For that purpose the method was well established in the United States in 1945. In the United Kingdom, where low-pressure thicker-walled cast-iron pipes were extensively used, very little cathodic protection was applied until the early 1950s. The increasing use of cathodic protection has arisen from the success of the method used from 1952 onwards to protect about 1000 miles of wartime fuel-line network that had been laid between 1940 and 1944. The method is now well established.
The most rapid development of cathodic-protection systems was made in the United States of America to meet the requirements of the rapidly expanding oil and natural gas industry which wanted to benefit from the advantages of using thin-walled steel pipes for underground transmission. For that purpose the method was well established in the United States in 1945. In the United Kingdom, where low-pressure thicker-walled cast-iron pipes were extensively used, very little cathodic protection was applied until the early 1950s. The increasing use of cathodic protection has arisen from the success of the method used from 1952 onwards to protect about 1000 miles of wartime fuel-line network that had been laid between 1940 and 1944. The method is now well established.
Cathodic protection can, in principle, be applied to any metallic structure in contact with a bulk electrolyte. In practice its main use is to protect steel structures buried in soil or immersed in water. It cannot be used to prevent atmospheric corrosion. Structures commonly protected are the exterior surfaces of pipelines, ships’ hulls, jetties,foundation piling, steel sheet-piling, and offshore platforms.
Cathodic protection is also used on the interior surfaces of water-storage tanks and water-circulating systems. However, since an external anode will seldom spread the protection for a distance of more than two or three pipe-diameters, the method is not suitable for the protection of small-bore pipework.
Cathodic protection has also been applied to steel embedded in concrete, to copper-based
alloys in water systems, and, exceptionally, to lead-sheathed cables and to aluminium alloys,
where cathodic potentials have to be very carefully controlled.
Source:http://www.npl.co.uk/upload/pdf/cathodic_protection_in_practise.pdf




